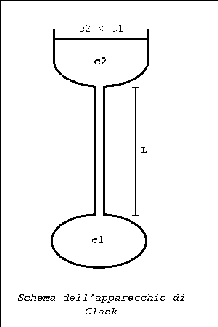
There are several methods to make the measurement of the diffusion coefficient for liquid solutions.
The apparatus consists of a very thin vertical glass capillary
which ends at the two extremities whith expantions as reservoir.
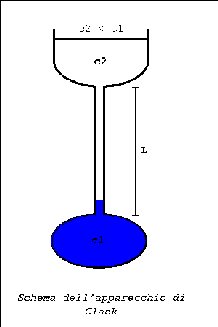
The lower reservoir is filled with the more dense solution, the upper with the less dense one ( usually the more diluite or pure solvent) to have no disturbance from gravity .
What does it happen filling the cell?
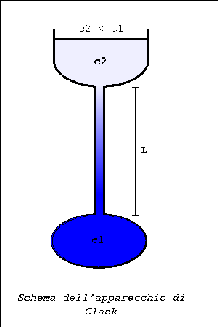
After reaching a stationary state, the flux of diffusing substance depends only on the concentration gradient which is constant in time.
(*)
This means that the concentration difference between two points, whose distance Dx is fixed, is a constant independently of the position of the two points along the capillary.
Upon reaching a stationary state, the flux of diffusing substance depends only on the constant concentration gradient

and the concentration in the capillary shows a linear profile.
The procedure is as follows: measurement of
the small concentration variations in the
superior reservoir, determination of the flux of the diffusing substance
J=dc/dT and derivation of the diffusion coefficient using the First Fick's law
equation (italian), with the constant concentration
gradient obtained by (*).
![]() CLICK HERE TO START THE INTERACTIVE APPLICATION
CLICK HERE TO START THE INTERACTIVE APPLICATION
![]() Go back to the Macroscopical Analysis
Go back to the Macroscopical Analysis
![]()