Quantitative Analysis
First Fick's law: kinetic point of view
Quantitative Analysis
![]()
Let's consider a cylinder filled with two solution of the same
substance.
Put it in a thermostate at constant temperature T.
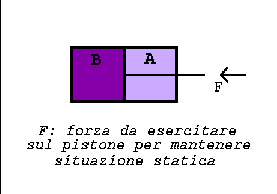
A piston which is permeable only to solvet molecules but not to that of
the solute divide the cylinder into two parts: A and B.
In A there is the less concentrate solution, in B the more concentrate.
Now let's try to understand the first Fick's law (italian) in the kinetic meaning.
The diffusion coefficient D gives the numbers of diffunding moles in a time unit unit of time, per a given area and per a given concentration decrease.
The motion of of a sinle particle do not obey to any rule and do not have a favourite direction.
Among the molecules in the two sides of the considered layer, half moves to the right and half to the left. As assumed, their number is bigger to the left than to the right, so a bigger number of them will move to right, the side of the more diluite solution, than to the other side. This process ends only when the concentration is equal everywhere inside the recipient: at that time all the movements compensate each other.
In B the solute has an higher concentration than in A: the force exerted on the piston is equal to the osmotic pressure difference times the area of the piston. So the partially permeable piston moves to the right filtering the solvent, till the solute concentration in the two chambers is equal.
The force which tends to equalize the concentration, in the two sections and therefore to make the solute diffuse, is due to the osmotic pressure.
The diffusion process can be stopped by applying from the exterior a force F equal and contrary to the force generated by the osmotic pressure difference.
Animation
Let now deffund the solute in a cylinder with no pistons, where concentration be bigger to the left than to the right.
The force which provoke the deffundent movement will be (italian)
Now we know the force which provoke the deffusing movement, we have to determine te resistance to this movement. Considering the Einstein model the resistance r will be given from the Stokes relation (italian).
In a unit of volume there are nN molecules of solute, where N is the Avogadro number, n the number of moles. The force k acting on these molecules will give them a velocity nN times lower. The force will be:
comparing (*) whith the first Fick's law (italian) we imediately observe that, apart of the volume, J = nv and dc/dx = dn/dx. Then, remembering the Stoke's relation (italian) :
(*)
With theorical arguments we arrive to an expression with which we can express the value of the diffusion coefficient vs temperature,viscosity, solvent and molecular radius of the solute.
The same relation permits to determine geometrical dimensions of the solute (italian) once determined the diffusion coefficient.
![]() Back to the Einstein's model
Back to the Einstein's model
![]()
![]() Notes
Notes
![]()